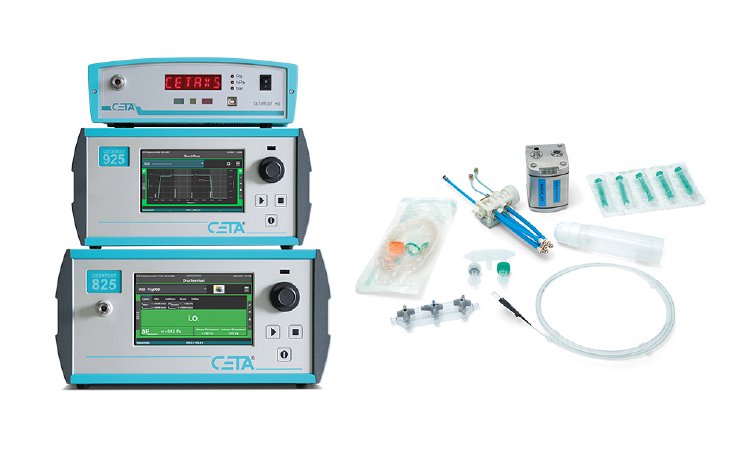
Why leak and flow testing is so important in medical technology
Medical devices – from disposable items to complex assemblies – must function reliably under real-world conditions. Small leaks or unexpected permeabilities can:
• compromise sterility – contamination due to the ingress of germs or liquids.
• impair the dosing accuracy of infusion and injection systems – incorrect flow rates.
• lead to malfunctions of tubes, valves, connectors, electronic devices, and display units.
Leak and flow tests are therefore an integral part of 100% end of line inspection and the validation of production processes. This allows defective products to be identified at an early stage, reduces recall risks, and supports compliance with regulatory requirements.
Typical medical devices for which leak and flow testing is essential
Examples of medical devices that have already been successfully tested with CETA testing devices and compressed air as the test medium:
• Luer connectors, plug-in systems, connectors – Leaks lead to loss of sterility or incorrect medication application.
• Infusion tubes / catheter connections – Flow testing ensures correct flow rates and detects partially blocked channels.
• Syringe bodies and pistons – Test for tightness and sliding behavior to guarantee dosing accuracy.
• Disposable filters and membrane components (e.g. respiratory systems) – Test for permeability and defined flow characteristics.
• Small-volume housings and connecting components (e.g. pump heads) – Functional reliability with complex geometries.
Different test methods are used depending on the test requirements: pressure decay measurement, pressure rise measurement, mass flow measurement, volume flow measurement.
Test devices for quality control using compressed air as the test medium
Compressed air is used as the test medium. It is dry, inexpensive, clean (no residues as with water), and allows short test times. These factors are particularly important when testing large quantities. The CETA test device range consists of leak testers, mass flow testers, volume flow testers, digital pressure manometers, and calibration standards. The range is complemented by suitable accessories for integrating the test devices into test benches.
All CETA test devices and calibration standards are supplied with an accredited and internationally recognised calibration certificate – compliant with DIN EN ISO/IEC 17025 – at no extra charge. In addition, the test devices come with a 3-year warranty with annual maintenance, which can be extended to 5 years.
About CETA Testsysteme GmbH
CETA Testsysteme has been developing and manufacturing industrial leak and flow testing devices “Made in Germany” for more than 35 years. The devices are integrated into production lines for 100% routine testing of products. They are used in industries such as automotive, medical technology, packaging, and electronics. Several thousand CETA testing devices are in use worldwide in production lines for quality control.
CETA at the MEDICA 2025 trade fair in Düsseldorf, Germany
At MEDICA 2025 (November 17-20, 2025) in Düsseldorf, the world's largest trade fair for the medical industry, CETA will be demonstrating live solutions for typical testing tasks in medical technology. CETA experts will be showing practical examples, explaining measurement principles, and advising on feasibility studies, integration into production facilities, calibration, and service. Interested trade visitors can find out about solutions for industrial leak and flow testing of medical devices on site.
CETA will give a presentation entitled ‘Leak and Flow Testing of Medical Devices in the Manufacturing Process’ on 19 November 2025. The presentation will take place as part of the stage programme at the joint state stand of North Rhine-Westphalia.
CETA at the MEDICA 2025 trade fair, November 17–20, 2025, in Düsseldorf, Germany
Hall 3, Booth C 80 – Joint community booth of the state of North Rhine-Westphalia
Further information
MEDICA 2025 trade fair
CETA exhibitor profile at MEDICA 2025
Homepage CETA