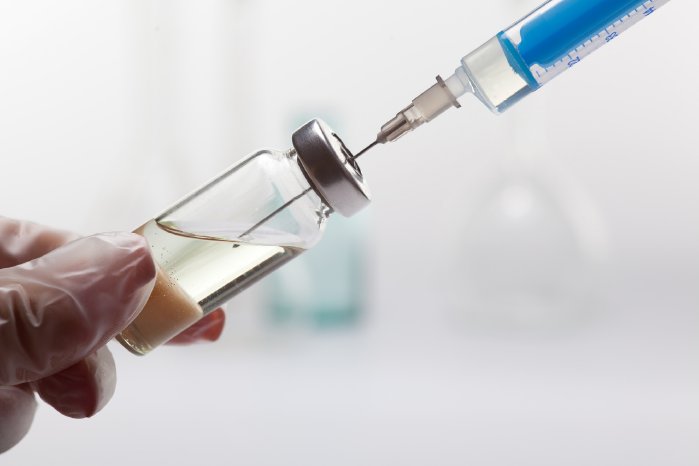
Cytotoxic drugs or cytostatics are drugs used to destroy cancer cells. They act by inhibiting cell division and in this way cause cancer cells to die. Whilst all dividing cells are affected, including those of healthy tissue, cancer cells often divide faster than normal cells, and as such the effect on normal cells is less pronounced and healthy cells will recover faster.
The drugs can be used to destroy tumours, boost the outcomes of surgery or radiotherapy, reduce metastases and alleviate cancer symptoms. Cytostatics can be effective outside the primary tumour and destroy small tumours that have not been detected in tests.
The use of cytotoxic drug treatment with different mechanisms of action has led to significant improvements in patient survival and a decline in mortality rates. This has been further enhanced by improved cancer screening and early detection, along with cause prevention by healthcare education.
The Tentamus Group is made up of class leading independent laboratories, providing high quality service and support to our clients worldwide. Our expertise covers a comprehensive and diverse portfolio of services to meet the fast-moving demands of the industry, through our dedicated laboratory locations in Europe, UK, Israel, China and the United States. Visit our website www.tentamus.com for more information.
If you have a requirement for testing of cytotoxic products, please contact one of our specialist GMP approved laboratories, who are specifically trained to handle cytotoxic products which are destined for market within the both the EU and UK.
Get in touch.