The pancreas is located behind the stomach, across the upper abdomen, and produces digestive enzymes and hormones. If a tumor forms there, rapid diagnosis and a quick start to therapy are crucial. This is because metastatic pancreatic carcinoma in particular is not considered curable. In this case, the tumor has already released cells into the blood that can form metastases elsewhere in the body. After surgical removal of the tumor, patients receive adjuvant chemotherapy to destroy any tumor cells still in the body. Nevertheless, pancreatic cancer often develops resistance to treatment. In addition, there are also targeted, personalized therapies that influence important signaling pathways of the tumor. However, no therapy has yet been established that is particularly promising. In addition, once resistance has developed, there are currently only a few possibilities to react.
For this reason, industry partners and researchers have joined forces to break new ground in a research project that is being implemented as part of the "Central Innovation Program for SMEs" funding program of the German Federal Ministry for Economic Affairs and Energy (BMWi; project form R&D cooperation projects; AiF Projekt GmbH). In the project, a novel screening tool is being developed: a drug screening platform. This will make it possible to determine the effect of therapeutic agents on tumors on a patient-specific basis at each treatment period so that the best possible therapy can subsequently be administered. The aim of the drug screening platform is to determine the best therapy option with the fewest side effects within a very short period of time for the patient concerned, in order to improve the treatment of pancreatic cancer and ultimately other tumors in the future.
Within the project, PreciPoint is developing a new fluorescence platform that will be integrated with the current M8 model. The M8+ will have the capabilities of the proven platform (fast scanning speed, instant access to data) and will be able to use it in two different application modes (brightfield and fluorescence) without any transition. The combination of brightfield microscopy and fluorescence technology allows the use of both immunohistological and fluorescent marker stained samples, which improves the specificity of image data used for diagnostics and research.
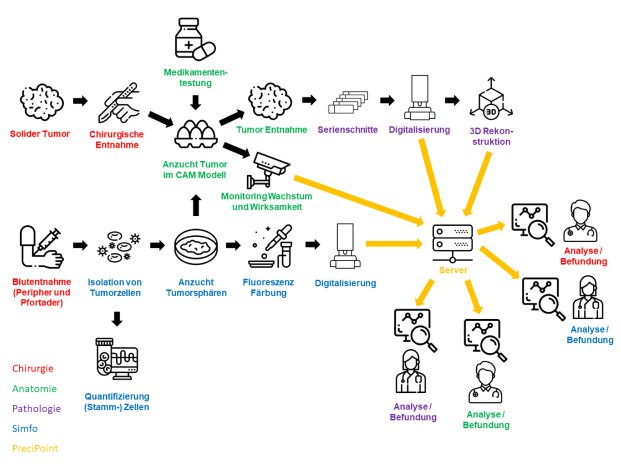
In addition, PreciPoint is developing an online platform that virtually links surgery, laboratory medicine, anatomy, pathology and basic science. Using the platform, metadata, diagnostic data and image data can be shared directly, allowing the different departments to collaborate quickly and effectively. With the development of new algorithms, structural analysis of the data and drug screening of the samples with certain characteristics are enabled.
The samples for the drug screen platform come from patients of the University Hospital Regensburg who want to participate in the study. Prof. Dr. Christina Hackl (Clinic and Polyclinic for Surgery, University Hospital Regensburg) coordinates the study-related process within the surgical department. They donate blood and tumor samples that are not used for diagnostic purposes. From the blood, tumor cells that the tumor has already released into the patient's bloodstream are isolated in Professor Katharina Pachmann's laboratory. Metastases can theoretically grow from these cells. The simfo GmbH from Bayreuth grows so-called tumor spheres (spherical cell colonies) from the cells. simfo GmbH already has many years of experience in the morphological, molecular biological and functional characterization of cells from solid epithelial tumors that are released into the blood.
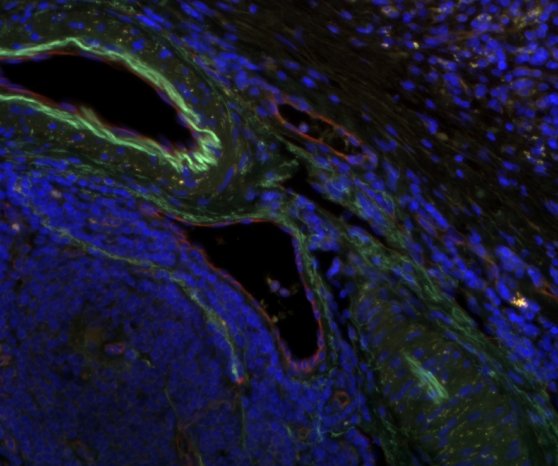
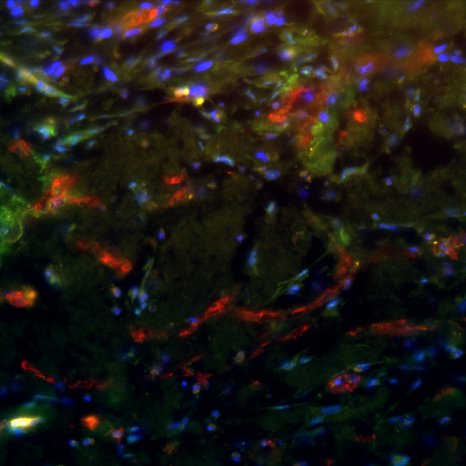
The collected tumor samples are cultured directly after surgery using a chorion allantoic membrane model (CAM model). The CAM is the membrane that forms the protective layer between the eggshell and the chick in a chicken egg; it can nourish tumor cells and tumor tissue like a placenta. Tumor spheres grown in simfo are also grown on the CAM membrane as so-called "patient-derived xenografts" (PDX). This step is performed in the research groups of Prof. Dr. Silke Härteis (Chair of Molecular and Cellular Anatomy), Prof. Dr. Thiha Aung (Chair of Molecular and Cellular Anatomy; Deggendorf University of Technology). The experimental setup allows tumor growth to be evaluated at any time. Thus, the efficacy and selectivity of established and also new therapeutics on the growth of tumor cells as well as on angiogenesis processes (formation of new blood vessels) and the ingrowth of the tumor into the surrounding tissue can be analyzed directly. Resistance to therapeutic agents can also be detected in this way.
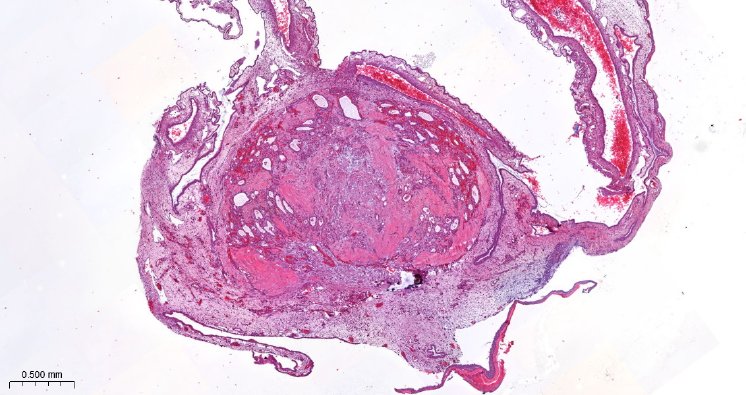
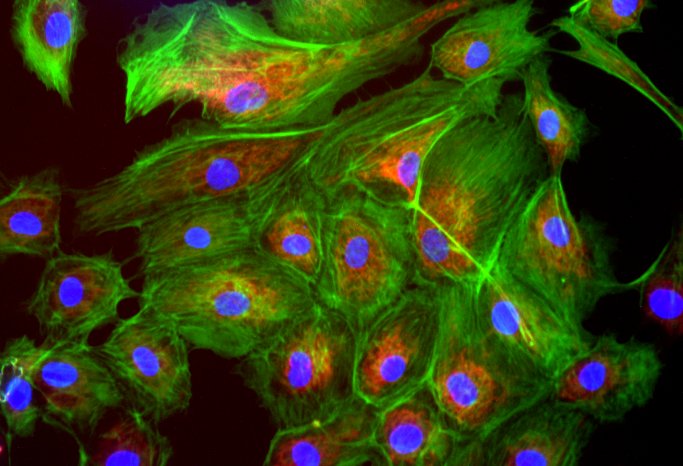
The exact characterization of the tumor tissue and the tumor cells takes place in part at the Institute of Pathology in the working group of Prof. Dr. Brochhausen-Delius through structural and ultrastructural analyses. There, histological, immunohistological and electron microscopic examinations are performed with the tumor spheres and tumor tissue. For this purpose, sectional preparations are made with the different samples from the CAM model. The samples are scanned with the M8 microscope (PreciPoint GmbH) to produce overview images of the slice preparations, so-called "Whole Slide Images" (WSI), for the analyses. Serial sections can also be used to perform a 3D reconstruction of the immunohistochemically stained blood vessels to more accurately visualize the vasculature and its branches. Finally, through the analyses, conclusions can be made about functional molecules, growth of the tumor and blood vessels to gain a deeper understanding of the tumor's blood supply and its makeup, also in comparison to metastatic tumors.