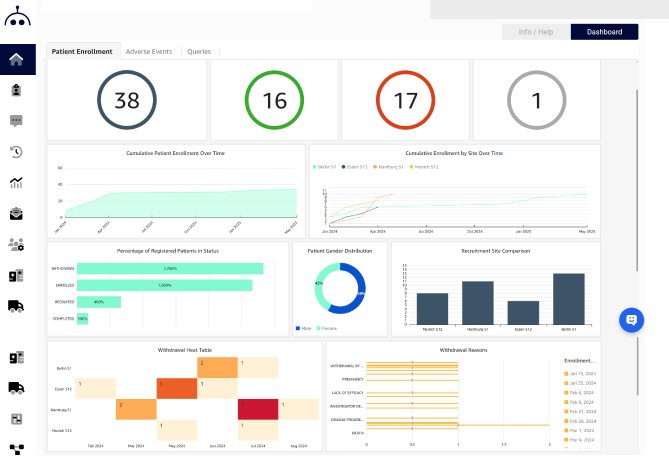
StudyMetrIQs delivers customizable, real-time dashboards, providing sponsors, CROs, and site teams the ability to track key metrics, monitor site performance, and explore operational data with ease. Whether you're focused on high-level KPIs or detailed site activity, StudyMetrIQs offers a flexible, intuitive environment to stay on top of every aspect of your study.
Key Features & Benefits:
Custom Dashboard Design
Create unlimited dashboards tailored to your layout, audience, and study needs.
Comprehensive Visual Options
Choose from 30+ chart types per widget, combine multiple visuals in one view, and add branding or labels to enhance clarity.
Embedded Experience
Access dashboards within your Marvin EDC instance, no additional logins or tools required.
Role-Based Access
Seamlessly integrated with Marvin's Roles & Permissions framework for secure and tailored visibility.
Predictable, Transparent Pricing
Flat monthly fees with no hidden infrastructure costs or data volume restrictions.
Expert Onboarding Support
Dedicated setup assistance ensures your dashboards are configured to your study’s unique needs.
Coming Soon:
Self-Service Editor
Empower users to build, publish, and share dashboards and no training required. Ad-hoc insights are just a few clicks away.
AI-Powered Insights (ML)
Automatically detect outliers, uncover hidden patterns, and highlight critical trends, helping teams act on the metrics that matter most.
“With StudyMetrIQs, we’re giving study teams a faster, smarter way to understand their data, without adding complexity,” said Manuel Neukum CEO at EvidentIQ Germany. “This is a major step forward for study oversight within our eClinical solutions.”
Ready to transform your study oversight? Contact us for more information info@evidentiq.com
https://xclinical.com/...