“While not fatal, recurrent syncope is quite dangerous and seriously compromises the patient’s quality of life,” commented international coordinator for the study Dr. Michele Brignole, Tigullio Hospital, Italy. “Previous studies have provided contradictory results regarding the benefit of cardiac pacing in syncope, and the current European Society of Cardiology (ESC) guidelines for this indication suggest that further study is necessary. The BIOSync CLS trial has the potential to clarify whether a dual-chamber pacemaker with the uniquely physiologic CLS pacing algorithm is able to detect and quickly neutralize syncope. I am very excited to begin this highly significant trial.”
The international, double-blinded multicenter trial will include 30 study centers in Italy, Spain, France, the Netherlands, Portugal, and Canada. Investigators will randomize 128 patients with a pacemaker indication for recurrent syncope into two groups. Patients in each study arm will receive a BIOTRONIK dual-chamber pacemaker with CLS turned either on or off. The trial’s primary endpoint is to compare the time to first recurrence of syncope between the active group (detection mode and CLS turned on) and the control group (placebo, detection mode only). There will be a follow-up period of two years; information regarding primary endpoint events will be evaluated via anonymous patient questionnaires gathered by independent personnel blinded to the randomization.
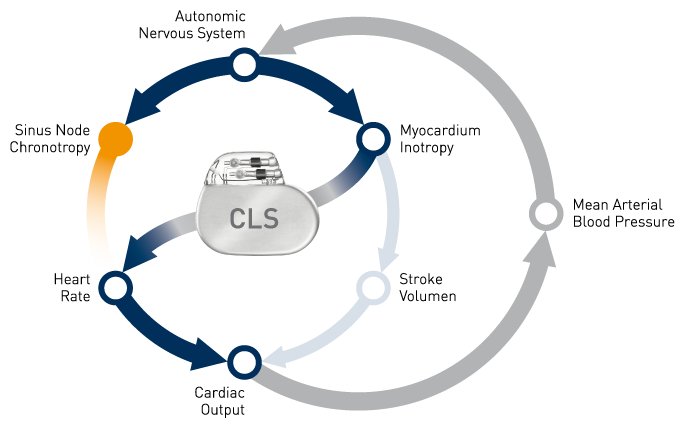
“A number of previous trials have shown that CLS is sensitive to contractility, or the self-contraction of the heart muscle.1,2,3 We hypothesize that pacemakers with CLS are uniquely capable of detecting the increase in contractility which can occur in the early stage of a syncopal event. They should therefore be able to initiate cardiac pacing at an earlier stage to prevent the sudden drop in heart rate which often causes syncope,” stated first implanter Dr. Marco Tomaino, Bolzano Hospital, Italy. “If our hypothesis is proven correct, implications for treatment indications and guidelines would be widespread.”
BIOTRONIK CLS rate adaptive technology is available in select pacemakers, implantable cardioverter-defibrillators (ICD), and cardiac resynchronization therapy (CRT) devices. CLS integrates into the natural cardiovascular control system and determines the appropriate heart rate based on intracardiac impedance measurements. These measurements reflect changes of the cardiac contraction dynamics in reaction to information coming from the autonomic nervous system. In doing so, CLS provides optimal heart rates under varied circumstances, even during acute mental stress. Several smaller studies have provided evidence that CLS might reduce syncope episodes in select patient groups.
“At BIOTRONIK patient safety and quality of life is central to everything we do. Syncope patients are a quite challenging patient population, with no other treatment options currently available and a lack of clear consensus among the medical community,” stated Albert Panzeri, Vice President at BIOTRONIK. “With the BIOSync CLS trial, we are doing our part to gather clinical evidence with the potential to make a huge difference in these patients’ lives.”
References:
1 Occhetta E, et al. Europace. 2004, 6(6)
2 Palmisano P, et al. Europace. 2012, 14(7)
3 Russo V, et al. Heart. 2013, 99(21)
For more information, visit: www.biotronik.com