Novalung announces receiving CE mark approval for their new pump-driven iLA activve® lung support system. It will be launched in Europe with immediate effect.
Respiratory support with iLA activve® enables a treatment environment with awake, mobile patients instead of sedated patients on invasive mechanical ventilation that causes further damage to the lung and other organs. The iLA activve® is thus a major step towards replacing invasive mechanical ventilation.
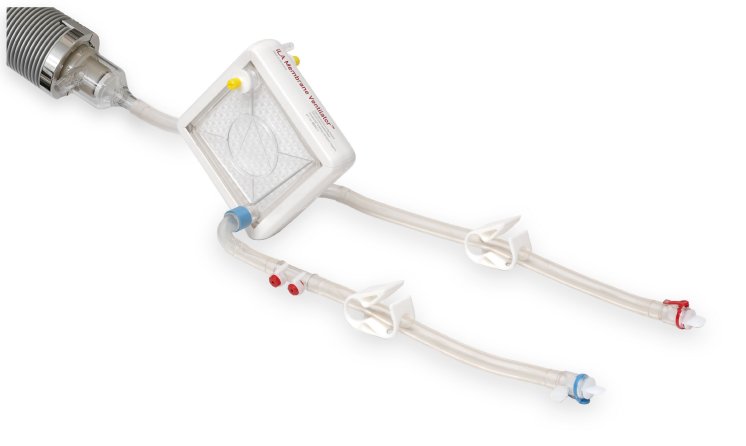
Different patient populations and types of lung failure place varying demands on optimal respiratory support. To cover all extrapulmonary support needs with one system platform, the iLA activve® has been developed as a flexible all-rounder. The iLA activve® with veno-venous cannulation covers the full range of respiratory support, from highly effective carbon dioxide elimination to complete oxygenation. Core elements are the iLA Membrane Ventilator®, the only gas exchanger specifically designed for long-term respiratory support, and the iLA activve® pump which surpasses all previously available blood pumps in its performance, broad range of blood flow, and blood protection characteristics.
The iLA activve® system has been specifically configured to allow patients to move about during respiratory support. Therefore all necessary components are mounted on an easily movable trolley. Furthermore the iLA Membrane Ventilator® and the iLA activve® pump are height-adjustable via a quick-release handle. “This allows physicians, respiratory therapists and nurses to adjust the system to the patient’s individual needs, whether the patient is in bed, sits up or even walks”, explains Dr. Georg Matheis, Managing Director of Novalung.
The range of applications of the iLA Membrane Ventilator® technology has broadened substantially. While severe acute lunge failure (ARDS) was the typical indication in the early days, today exacerbations of chronic lung disease (COPD) comprise a growing application of the iLA Membrane Ventilator®. Novalung will launch several additional products for the iLA activve® platform throughout this year.