The upgraded certification means that in the future seven FITBONE® nails in the TAA series will bear the CE mark. This symbol is a mandatory marking for medical devices and a declaration of compliance with numerous legal and standard requirements, which are verified by a notified body in the framework of a conformity assessment procedure. As Roman Stauch, Managing Director of WITTENSTEIN intens GmbH, explains, the very comprehensive safety measures in place for the FITBONE® System were an important aspect for TÜV Süd Product Service GmbH of Munich, the certification body responsible: “The CE marking requirements were met in every respect and without exception. Under the European Economic Community’s Active Implantable Medical Devices Directive, therefore, FITBONE® has various warranted characteristics with benefits for patients and attending physicians alike: among other things, the intramedullary lengthening nail is guaranteed to be biocompatible and the risk of infection reduced to a minimum. We also commit ourselves to keeping a close watch on our manufacturing processes – throughout the entire product lifecycle.”
The upgraded certification applies to the following FITBONE® nails: TAA1140-F-205, TAA1180-F-245, TAA1380-F-245 (for femur) and TAA1140-T-205 and TAA1160-T-225 (for tibia).
New receiver function improves safety during the leg lengthening process
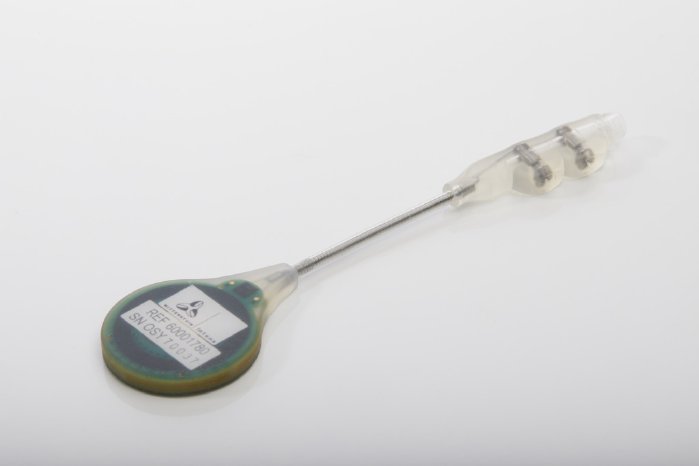
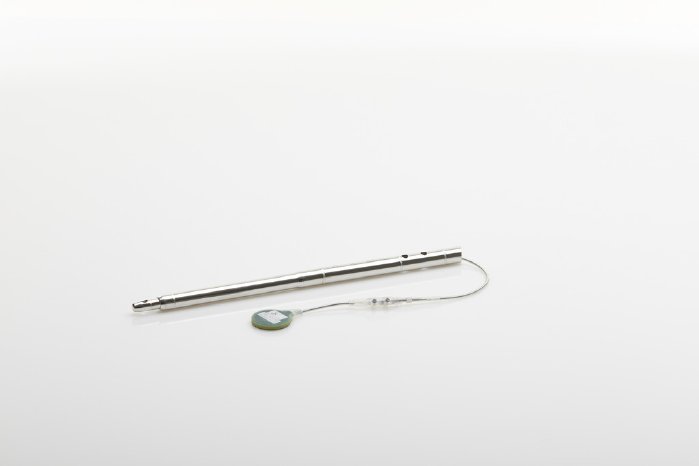
The completely redesigned receiver, which is now about 45 percent smaller in size, is another innovative feature of FITBONE®, the intramedullary lengthening system. The receiver has simultaneously been enhanced with a retraction function, which lets the attending physician reverse any actions inadvertently carried out by the patient. For example, if the lengthening nail is accidentally extended too far, the physician can in future retract it again if necessary.
Maximum comfort during leg lengthening
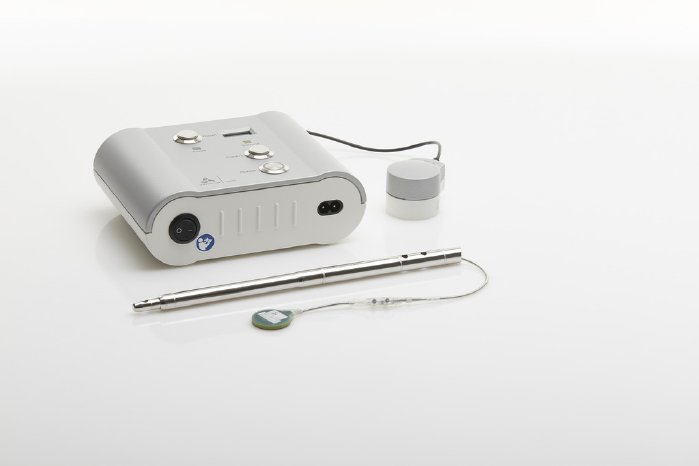
FITBONE®, the smart intramedullary lengthening nail, is a unique mechatronic system for compensating leg length discrepancies. It can simultaneously be used to correct deformities of the femur and tibia. FITBONE® is implanted in the bone in a minimally invasive surgical procedure while the receiver is placed directly beneath the skin. Patients can control the distraction process themselves three times a day using the external FITBONE® Control Set. The visual and audible impulses for monitoring the implant system deserve particular mention here because they help patients perform the distraction safely, independently and in a controlled way.
Thanks to the FITBONE® app and full treatment documentation, the lengthening process can be constantly monitored. Both the procedure itself and its performance at the programmed times can be recorded by the patient in this way. The FITBONE® app also provides patients with other valuable information. This enhanced functionality – unprecedented with implants of this kind – makes the leg lengthening process much safer and easier to control.