The official Swissmedic Laboratory, a member of the OMCL (Official Medicines Control Laboratories), has been involved in the examination of Sartan drugs, concerning potential impurities with the Nitrosamine NDMA (N-Nitrosodimethylamine), since July 2018.
The investigations and actions were triggered by market recalls of drug products (DP) from several DP manufacturers, on account of impurities with NDMA in DP containing Valsartan. The affected batches were delivered by a Chinese active pharmaceutical ingredients (API) manufacturer, who stated, changing their synthesis process during the production of the API, has been the reason for contaminations of Valsartan with NDMA.
During worldwide investigations and testings of relevant APIs from other manufacturers there have been inacceptable findings of NDMA, but also of an additional Nitrosamine NDEA (N-Nitrosodiethylamine).
Therefore all, on the Swiss market available, DP containing the APIs Candesartan, Irbesartan, Losartan, Olmesartan and Valsartan, have been tested on NDMA by the Swissmedic until November 2018. These APIs contain a specific ring system, the so called Tetrazole ring, which causes the impurity with NDMA, at specific conditions of synthesis, during their production. Other Sartan containing APIs, such as Azilsartan, Eprosartan and Telmisartan, are not affected. Further investigations on NDEA are carried out by the Swissmedic at present.
According to the Swissmedic, all Swiss admitted applicants with DPs on the Swiss market, containing the above mentioned and relevant APIs, have to carry out testings on NDMA and NDEA on every API batch received.
In December 2018 the Swissmedic has released a limit testing method of NDMA and NDEA in Sartan drugs and APIs via GC-MS, with a suggested limit testing of < 300 ppb for NDMA and < 177 ppb for NDEA. Official transition periods of compliance, have been communicated individually to the affected DP manufacturers.
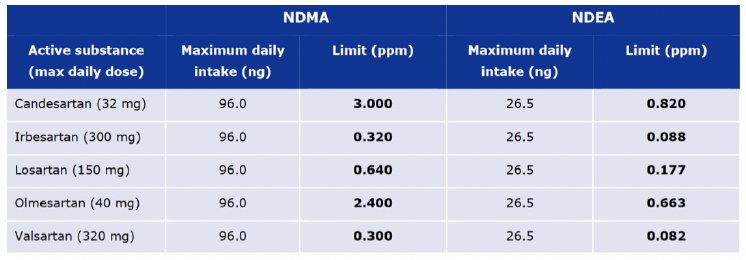
The European Medicines Agency (EMA) has released official temporary limits for NDMA and NDEA impurities, based on the maximum daily intake for each relevant impurity derived from animal studies (Tab.1). Within a transition period of two years, DPs containing impurities above those limits of a single impurity, or DPs containing detectable traces of both impurities, will lose their marketability within the EU. After the transition period of two years the limit on DPs for impurities of NDMA and NDEA will be < 0.03 ppm.
Dominique Weiss, Managing Director of Tentamus Helvetia, says, “The quality understanding of products ‘made in Switzerland’ is outstanding. For Tentamus, as one of the global leaders in high end quality testing services for the life science industries, it is crucial to provide extensive solutions for challenging analytical cases. For our understanding of excellent customer service, it is not enough to offer an analytical method which is in compliance with the limit testing method. Our customers, most likely, will implement internal specification limits, which will undercut the official limits of compliance with a safety range. To meet their expectations and to assist in their daily QM/QA routine, we have developed a quantitative method, with which we are able to determine the amount of NDMA and NDEA simultaneously in DPs and APIs.”
Dr. Jochen Kolb, Managing Director of BLS-Analytik mentioned additionally: “We are happy to contribute to the control and safety of drug products in Switzerland and the EU with this new established GC-MS method in our company. We successfully implemented a liquid extraction method with a subsequent GC-MS measurement based on the Swissmedic method. Therefore, we are able to measure NDMA and NDEA in a short time and in low concentrations on a specific mass fragment detection. An extension of the method to other desired matrices is possible by an adoption of the extraction process and a validation run for new materials.”
About Tentamus Helvetia
Newly founded in June 2018, Tentamus Helvetia is focusing on the acquisition of new customers within the food, feed, pharmaceutical, medical and cosmetical businesses, building a reputation in the industries for quality, compliance, and customer service. Within the global network of the Tentamus Group they provide the exemplary contract analytical laboratory services that local, national, and international clientele have come to expect from a leading laboratory group. For further information please visit www.tentamus.com
About BLS Analytik
Since about 30 years we are working for many pharmaceutical and food companies.
We are a chemical and analytical laboratory and consulting partner for any concerns on quality control, analytical method development and analytical method validation of pharmaceutical products, medical products and dietary supplements.
Services of BLS-Analytik:
- Quality control of pharmaceutical products, medical products and dietary supplements
- Analytical development and validation
- Release- and stability testing
- GMP compliant analytical services and documentation