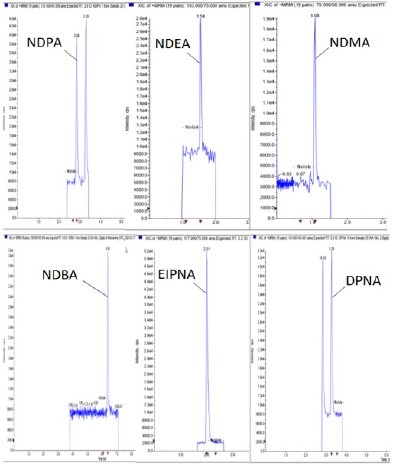
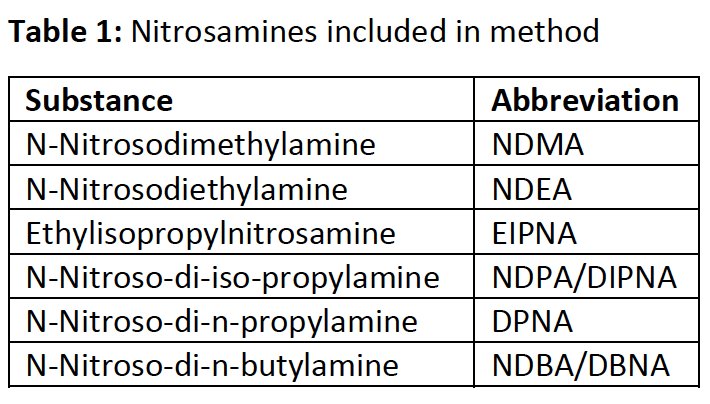
Based on a CVUA Karlsruhe method, DSI-pharm recently implemented a generic LC-MS/MS method with limits of quantification (LOQ) between 1 ng/mL and 5 ng/mL for 6 different nitrosamines including NDMA and NDEA (current spectrum of nitrosamines contained see Table and Figure 1). Samples of drug substances and products are tested for nitrosamine traces after being diluted in a solvent using liquid chromatography. This is coupled with mass spectrometry using atmospheric pressure chemical ionization (UHPLC-APCI-MS/MS) in multiple reaction monitoring mode (MRM).
The generic LC-MS/MS method can be easily adapted to different drug substances and products if necessary. The method must then be validated under GMP for each product. The final LOQ´s will range between about 0,010 ppm and 0,050 ppm depending on the nitrosamines and the required sample dilution. Upon request, further nitrosamines of interest can be implemented into the method.
DSI-pharm is highly experienced in trace and residue testing in food and pharma products and can offer you attractive turnaround times – also for method development and validation.
Do you have further questions about the analysis of nitrosamines?
Please contact our expert Dr. Serap Acikgöz.