Dr.-Ing. Hermann Monstadt, Managing Director at phenox GmbH, comments, "the pREset LITE has been developed to enable physicians treating ischemic stroke even in the very distal and therefore very sensitive area of the brain. It is based on the design of the pREset thrombectomy device which has proven its high clinical effectiveness in many hundred cases." With the new pREset LITE, the pREset, BONnet and BONnet short devices phenox GmbH now offers the broadest range of thrombectomy devices for the treatment of ischemic stroke.
"Stent Retrievers have been revolutionizing acute stroke treatment. The latest innovation on this field is undoubtly the pREset LITE, a three and four mm stent retriever that navigates through a 10-microcatheter," Dr. Vitor Mendes Pereira, Head of Interventional Neuroradiology at University Hospital of Geneva, Switzerland says. "It significantly improves the access to distal lesions, improves aspiration capabilities from proximal balloon guiding catheter or distal access catheters while keeping the same effectiveness on retrieving the thrombus from the occluded vessel. It is impressive how the technical evolution is pushing forward the acute stroke therapy field. Great new tool!"
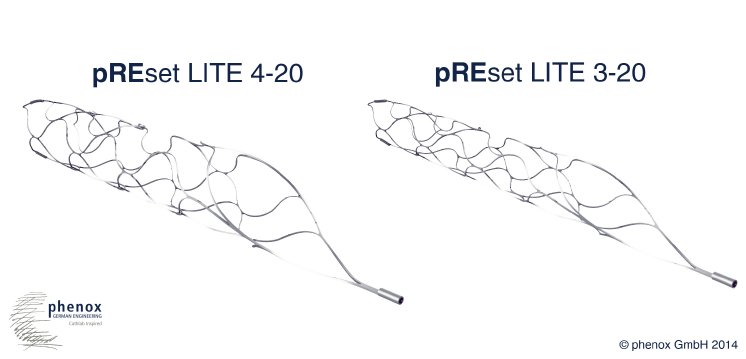
Like the phenox pREset thrombectomy device the pREset LITE has a proximal closed "ring" design. This ensures a stable opening, reduces tapering when withdrawn and provides optimized distribution of radial force. Together with its helically shaped slit the pREset LITE facilitates the safe and effective treatment of ischemic stroke. The pREset LITE is available in two sizes with diameters of three and four mm, each of 20 mm length. To ensure access to small and distal vessels both versions are compatible with 0.0165" ID microcatheters (e.g. Boston Scientific Excelsior SL-10, Codman PROWLER 14) and can be used in minimum vessel diameters of at least 1.5 mm.
According to Mr. James Lago, Consultant Vice President International Marketing & Sales at phenox GmbH, "the pREset LITE 4-20 is the first 4mm device for the intracranial retrieval of thombi that can be used in such small catheters". Dr. Wiebke Kurre, senior physician at Katharinenhospital in Stuttgart, Germany, comments: "vessel occlusion often extends far distal in the cerebral vasculature. Critical decisions have to be taken whether to proceed with the procedure accepting a significant risk of hemorrhage or to stop accepting incomplete recanalization and worse functional recovery. The low profile, low radial force pREset LITE allows for safer catheterization and thrombectomy in small vessels. Clincal experience with pREset LITE convinced me and I would not want to miss this device in my stroketoolbox." The pREset device has just further proven its safety and efficacy in a review of 271 consecutive cases.*
*Kurre et al. (2014): Clinical experience with the pREset stent retriever for the treatment of acute ischemic stroke - a review of 271 consecutive cases. Neuroadiology, March 2014. Berlin, Heidelberg: Springer.