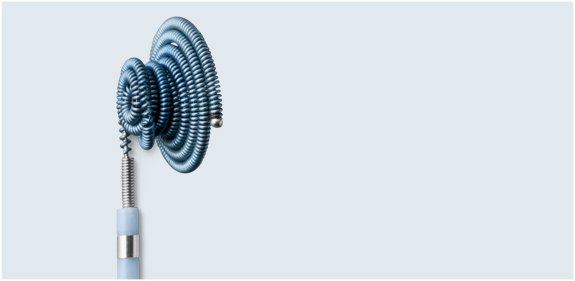
A Phase II Clinical Study was conducted with 378 subjects enrolled in 15 study centers throughout the U.S. under an Investigational Device Exemption (IDE) to establish a reasonable assurance of safety and effectiveness of the Nit-Occlud® PDA. Favorable results meeting all objective performance criteria from this clinical study were the basis for the premarket approval decision.
Aurel Schoeller, CEO of the pfm medical group, is pleased with the successful product introduction: „Nit-Occlud® PDA is one of our main innovative products. Its FDA approval for us represents a key step towards achieving market share in the US“, he said.