Digital partner management using NIC-place
Our pharmaceutical logistics customer relies on its network of service providers with various installed telematics systems to carry out the transports. Using NIC-place's user-friendly digital invitation and onboarding processes, over 80 of its subcontractors have already been integrated across Europe and around 4,000 vehicles activated online to ensure general access to telematics data. During the onboarding process, the transport service providers agree to the temporary data transfer of position and temperature data during the actual transport. NIC place controls the data transfer in this phase in accordance with data protection and compliance requirements.
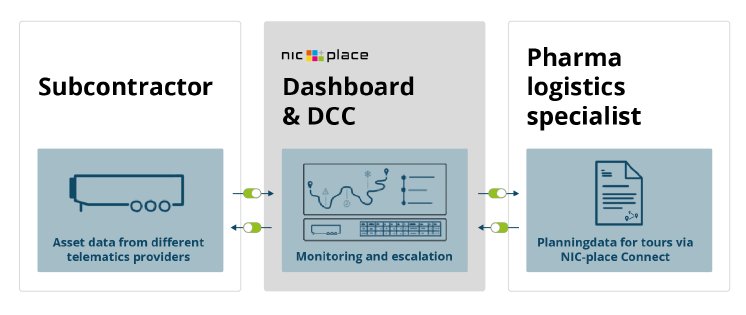
Linking of systems and operative procedure
The connection of the customer system with NIC-place serves as the basis for the automated data transfer and is done via the standardized NIC-place Connect interface. This allows for the exchange of information between the leading TMS planning and order management system of the pharmaceutical logistics company and our logistics solution as data aggregator of the telematics systems from the TSPs without double inputs.
As usual, the pharmaceutical logistics company plans the transport in its own system. Then, the planned tour is transfered to NIC-place including information about which subcontractor will be involved. As a result, the NIC-place system triggers a request to the known service provider to confirm the order and at the same time to assign the vehicle registration number that will operate the transport digitally. Now the planned tour can be technically activated by our logistics solution according to the desired parameters. All bundled tour information is then available to both the pharmaceutical logistics company and the service provider in both systems.
Real-time monitoring of transports by NIC-place
The monitoring of assets or transports as well as the data transfer to the pharmaceutical logistics company starts and ends POI-based. Depending on the desired trigger, the tour will automatically begin and end based on the entry and exit of the planned POI addresses. During transport, NIC-place monitors temperatures, reefer status, alarm codes, setpoints and GPS positions and escalates deviations from the target immediately to the customer system and the transport service provider. In addition, calculated arrival times (ETA) help to improve the flow of goods. Finally, end-to-end documentation of the transport process is made available directly to the relevant parties upon arrival by NIC-place; manual processes are eliminated. Among other things, this enables efficient unloading processes by offering insight into the status and condition of the goods and helps our customers to make decisions in real time.
Simple scaling
The basic setup of the pharmaceutical project can easily be extended to other business areas of the customer, such as the visualization of high-security, chemical or mineral oil transports.