Arm exoskeletons and virtual reality unite to help stroke patients move again
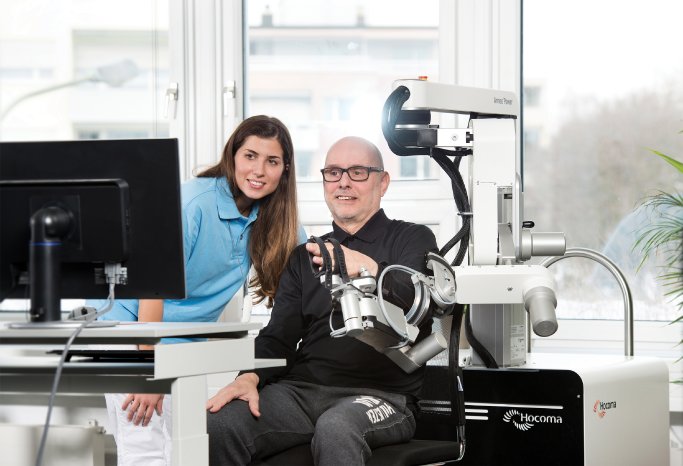
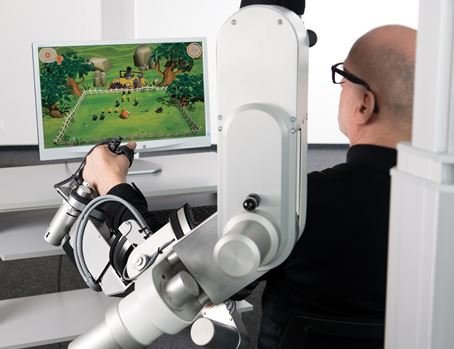
ArmeoPower enables highly intensive arm rehabilitation for early-stage patients even before they develop active movement. Research has shown that highly intensive, repetitive task-oriented movements enhance the neural plasticity of patients who suffered strokes, traumatic brain injury or neurological disorders. The ArmeoPower robotic therapy leverages these findings to help patients regain their arm and hand function by combining devices supporting the patients' movement with fun exercise games in virtual reality which motivate them towards higher active effort.
The new ArmeoPower takes upper extremity rehabilitation to a new level by introducing the integrated robotic hand module ManovoPower. The device supports the complete movement chain, offering simultaneous therapy all through from shoulder to fingers. The new ArmeoPower also includes a completely new software that increases the patient's motivation: "It provides hospitals with an enriched environment to train and assess even severely impaired patients with much higher intensity, accuracy and reproducibility than what is possible in today's clinical setting. The new therapy planning and reporting enable clinicians to tailor the therapy to each patient, thereby keeping them challenged and motivated", says Dr. Vaclav Potesil, Armeo Product Manager at Hocoma.
Renowned clinics from USA and Europe as first pilot users
Seven of the most innovative hospitals in USA and Europe already installed the exclusive pilot series of the new ArmeoPower to provide the first experience with the successful integration of the medical device into clinical routine. Dr. Alberto Esquenazi, pilot user of the ArmeoPower and Department Chair and Chief Medical Officer at MossRehab (Philadelphia, USA), has evaluated the new medical device and is convinced of its success: "We know that patients with hemiparesis will greatly benefit from integrated movement rehabilitation techniques, but we had no concrete options for this approach so far. But now, ArmeoPower offers us for the first time a technology that enables us to achieve this goal by intelligently supporting the complete movement chain from the shoulder to the fingers."
In addition to Moss Rehab (Pennsylvania, USA), the rehabilitation centers Sheltering Arms (Virginia, USA) and Dignity Health-St Rose Dominican (Nevada, USA) are part of the exclusive pilot group using the new ArmeoPower in the USA. In Europe, pilot customers include Kliniken Valens (Switzerland), Casa di Cura Habilita (Italy), SRH Bad Wimpfen (Germany) and Le Normandy (France). The first series device batch will be shipped to customers starting July 2015.
About the Armeo Therapy Concept
The Armeo Therapy Concept includes four distinct products for upper extremity neurological rehabilitation, which cover all stages of the recovery process, from the most severely affected early-stage patients to long-term rehabilitation in the out-patient settings. All Armeo products combine arm-weight support in a large 3D workspace with motivating game-like exercises and assessments to track the patient's progress.
Product Disclaimer
All Hocoma products are medical devices and must be used in strict adherence to the User Manual; failure to do so may result in serious personal injury. It is strongly recommended that you regularly consult Hocoma's website (www.hocoma.com/legalnotes) for the latest available information. Please contact Hocoma in case of any questions.
Use only under the supervision of qualified medical personnel. However, certain Hocoma products are marketed for home use and must be strictly used according to the recommendations of your medical care provider who is knowledgeable about your specific needs. Consult the User Manual and Hocoma's website (www.hocoma.com/legalnotes) for appropriate product designation. Failure to obtain and follow the recommendations of your medical care provider may result in serious personal injury.
This information provides details about medical products which may not be available in all countries and may not have received approval or market clearance by all governmental regulatory bodies throughout the world. Nothing herein should be construed as a solicitation or promotion of any product or of an indication of any specific use for any product which is not authorized by the laws and regulations of the country where the reader of this information resides.