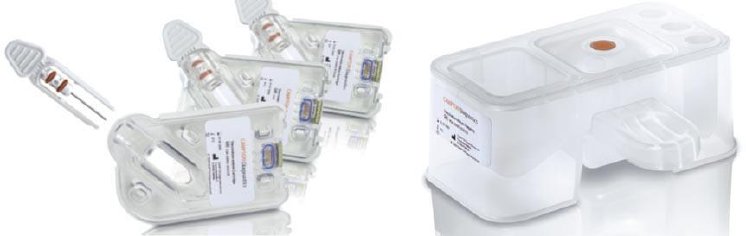
Initially, Gerresheimer provided the customer with several concepts for both the test cartridge containing the analyzer’s innovative biochip and the reagent block, from which up to four test fluids (reagents and sample diluents) are supplied and waste products are purged after testing.
For the final design of both components, elements of various drafts were combined and optimized. In developing the test cartridge, the initial focus was on improving the ergonomics of the glass capillary holder, which is where the patient’s blood sample is stored. A corrugated finger rest and two extended guide rails make sure that the delicate capillaries can be inserted safely into the cartridge.
Two O-rings also ensure the glass capillary tube is sealed in the cartridge. The cartridge itself was rounded to simplify production using injection molding. Ultra-transparent COP plastic was selected as the material for parts of the cartridge so that the user can check to make sure the capillaries are filled completely before inserting them into the test unit.
In designing the reagent block, Gerresheimer reduced and standardized the thickness of the block walls to achieve the highest possible stability and production speed with the lowest possible material Costs.
CAMPTON Diagnostics selected Gerresheimer as their development partner because the company offers agile project handling and can quickly and flexibly implement changes.
“We are well positioned to work both with start-ups and large pharmaceutical companies,” explains Manfred Baumann (Global Executive Vice President Sales & Marketing Administration & TCC). “Thanks to our multi-stage Gx phase model, we can optimally adapt to the needs of our customers. For start-ups especially, fast availability of functional prototypes or small series is critical for clinical testing.”
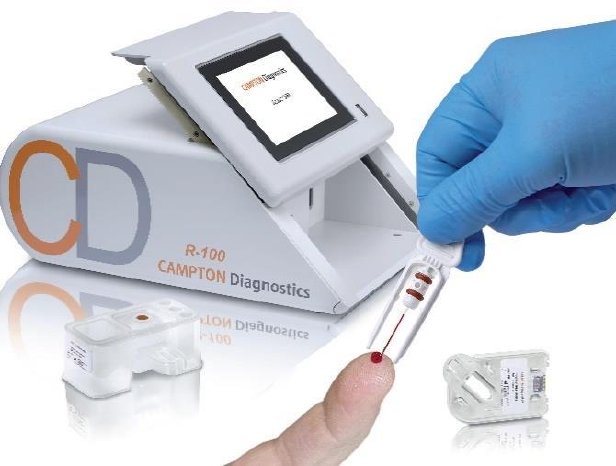
For the blood test, small amounts of capillary blood are taken from the patients, for example from a drop of blood. The blood is drawn by a glass capillary, which is inserted into the test cartridge. Next, the cartridge and reagent block are inserted in the unit. On the cartridge biochip, disease-specific biomarkers in the blood react with the corresponding antibodies on the chip. With the unit, indications of various infections and types of cancer or autoimmune diseases can be identified right at the point of care, depending on the test cartridge used, and samples no longer have to be sent to the lab and await analysis.
About CAMPTON Diagnostics
CAMPTON Diagnostics GmbH was established in 2016 as a spin-off of the Fraunhofer Institute for Silicon Technology (ISIT) in Itzehoe, which it maintains a strategic technology partnership with. The company develops and markets complete measurement systems for point-of-care diagnostics. CAMPTON Diagnostics combines biotechnological and engineering expertise under a single roof.
More information at www.campton-diagnostics.com