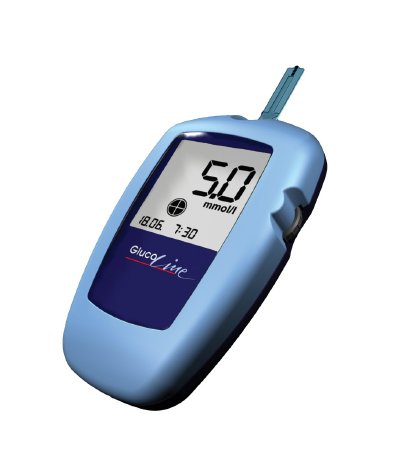
If the measurement was proceeded before or after a meal, if the patient was at rest, sick or active, when and which kind of insulin was supported - all of these information are very important for a proper diagnosis, and can now be entered quickly and easy in the electronic diary of the new GlucoLine devices from EKF-diagnostic, Germany. As an additional unique feature, the data in the device memory can be sent by mobile phone in an encrypted online-database, accessible for the personal doctor to give medical support whenever and wherever it is requested by the patient.
Berthold Walter, CEO of the EKF-diagnostic GmbH: "As an established manufacturer of clinical lab analyzers, we’ll implement the high quality standards of our products also in best usability for private users. Accurate measurement results are in our focus as well as comfort handling and convenient availability of health-related data."
Introduced at MEDICA, the new device series is represented by three different models: The standard "GlucoLine"-device is featured with Bluetooth and scroll wheel, whereas the "GlucoLine Basic" fits the demands of budget customers. Specialized for the clinical use, the "GlucoLine Manager" offers manifold options as Barcode reader, Bluetooth networking, graphical display and docking station, corresponding the future requirements in professional patient management.