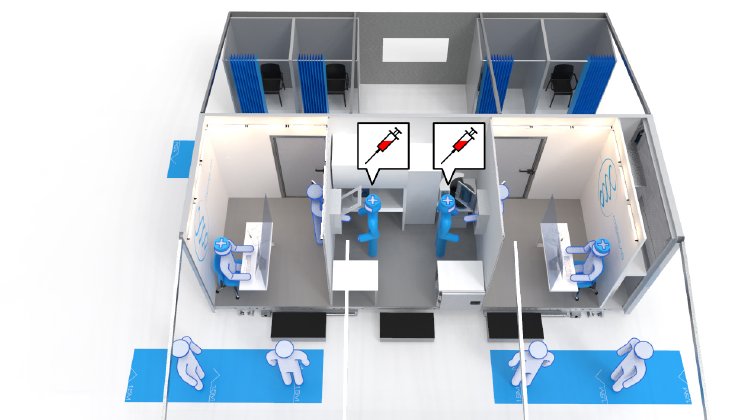
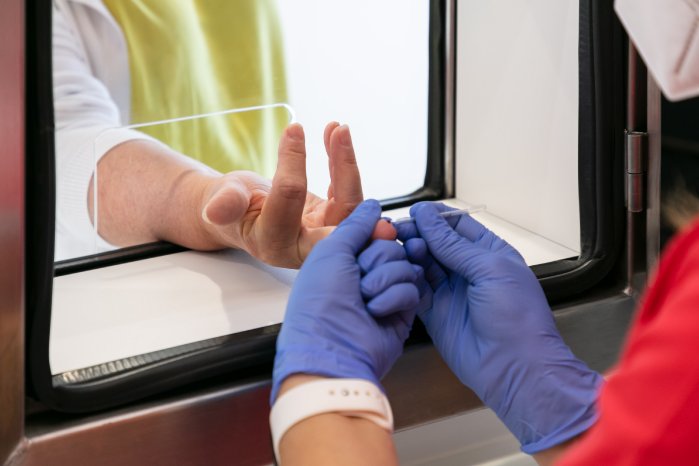
This type of mobile testing for the first time offers the possibility of testing larger groups of people on company or public-authority premises and obtaining the results shortly afterwards, thereby providing certainty as to whether anyone has been infected, and if so, who is infected, who is not infected and who is acutely infected. The tests are intended to prevent individual work absences through to complete production stoppages, and to provide the certainty that no infected employees are working. The intention is also to identify infected persons/groups of persons at an early stage. The fact that samples are taken under the same medical, spatial and technical conditions means that the results are comparable.
The laboratories are to be used not only for vaccinations and blood sampling, but also for major events and disaster-relief operations where laboratory capacity and treatment rooms are needed quickly, and can be technically upgraded to security level S3 in order to test for "undefined pathogens" such as viruses, bacteria, fungi and parasites.