“As we collect results at each stage of the BIOSOLVE studies, we are given increasing reason to believe that Magmaris will continue to be heralded as an effective solution in the long term,” stated coordinating clinical investigator Dr. Michael Haude, Lukaskrankenhaus, Neuss, Germany. “With no reported instances of scaffold thrombosis, BIOSOLVE-II results confirm that the magnesium scaffold is a safe solution for patients even after the scaffold has been completely resorbed, differentiating it from polymer-based scaffolds.” During his presentation, Dr. Haude showed that there was 0% definite and probable scaffold thrombosis with Magmaris at six, 12 and 24 months across the cohort of patients with de novo coronary artery lesions.
Dr. Haude also presented the pooled six-month data of the BIOSOLVE-II and BIOSOLVE-III studies. The combined results demonstrated consistent and low target lesion failure at 3.3% and no incidence of definite or probable scaffold thrombosis, further confirming the safety and efficacy of Magmaris. Clinical follow-up for BIOSOLVE-III will continue for up to three years.
“As the first clinically-proven resorbable magnesium scaffold (RMS), it is essential to generate positive long-term clinical evidence to reinforce physician confidence in Magmaris,“ said Dr. Alexander Uhl, BIOTRONIK Vice President Marketing Vascular Intervention. “We intend to continue to invest in this new proven technology in order to provide patients with the safest and most effective care. Corresponding with this, we are pleased to announce that BIOSOLVE-IV is underway, with over 200 patients currently enrolled for Magmaris implantation. This global study will include more than 1,000 patients with a five-year follow-up for longer-term evaluation of the magnesium scaffold.“
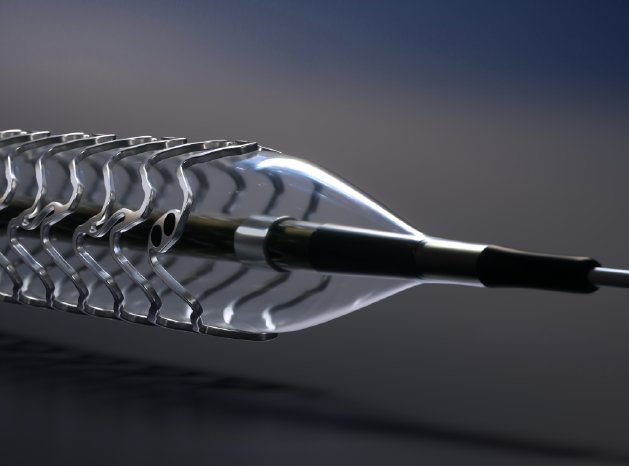
About Magmaris
Magmaris is a limus-eluting bioresorbable magnesium scaffold exclusively available from BIOTRONIK. Due to the scaffold’s magnesium backbone, it offers novel benefits that only a metallic scaffold can offer such as desired deliverability, strong radial support and a fast resorption time of approximately 12 months. In addition, the proven BIOlute coating, consisting of a limus drug and an excipient, ensures controlled drug release to inhibit cell growth similarly to Orsiro, BIOTRONIK’s hybrid drug-eluting stent.
Twitter: @BIOTRONIK_News
LinkedIn: www.linkedin.com/company/biotronik