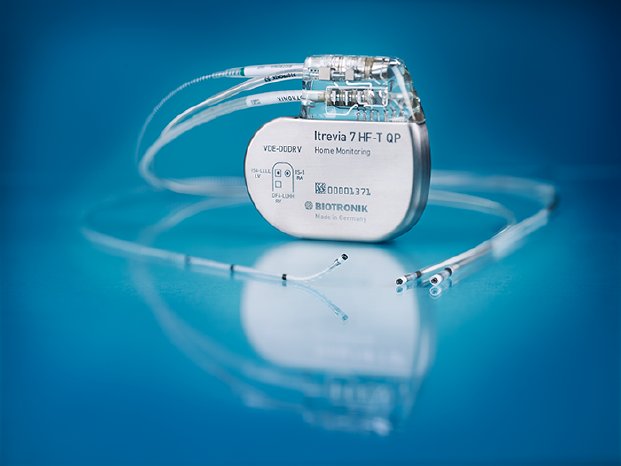
BIOTRONIK, the world’s leader in MR conditional cardiac devices, has announced that its Itrevia 7 HF-T cardiac resynchronization therapy defibrillator (CRT-D) has now been approved for full-body MRI scanning in Japan.
The device was launched in Japan in July of last year, and all already implanted devices have been deemed safe for full-body MRI scans at 1.5 tesla strength. Meaning, physicians can take MRI images of the heart area of these device patients—an unprecedented aid when treating this patient group. This is the first and only CRT-D in Japan with such conditions.
Dr. Seiji Takatsuki, Associate Professor of Cardiovascular Medicine at Keio University Hospital, said the announcement marks a huge leap in treating CRT-D patients with comorbidities. “In addition to having indispensable diagnostic information in the field of cerebrovascular treatment and orthopedics, MRI offers extensive diagnostic cardiovascular information. Many CRT-D patients undergo a cardiac MRI (CMR) before implantation to assess the late gadolinium enhancement. However, until now CMR after CRT-D implantation was not possible, thus no assessment on the presence or absence of any changes could be achieved. Now with the condition change, post-operative cardiac MRI examination has become feasible with the Itrevia 7 HF-T series.”
MRI offers very detailed images, so it is the method of choice for the diagnosis of many injuries and body conditions. Until recently, patients with cardiac devices were denied any MRI access due to the potential hazard the strong magnetic fields and radio waves would have on cardiac devices and leads. However, many of these patients will need an MRI scan within their lifetime.[1]
Addressing this issue through design and scrupulous testing, BIOTRONIK developed its ProMRI technology and now boasts the world’s largest portfolio of MR conditional devices and leads. Japan is the world’s largest MRI market with more MRI scanners per capita than any other country: approximately 47 registered machines per one million people[2]. Itrevia 7 will allow more patients access to this superior diagnostic tool, even in the cardiac area.
“We’re determined to help our patients live normal lives,” commented Jeffrey Annis, Managing Director BIOTRONIK Japan. “Having access to MRI scanning is part of that. Physicians have long asked for MRI access in the cardiac area of device patients and we are answering that call.”
For more information, visit: www.biotronik.com
References:
[1] Kalin R and Stanton MS, Pacing Clin Electrophysiol. 2005, 28 (4).
[2] OECD Health Statistics 2013 http://dx.doi.org/10.1787/888932917275.