“Now that clinical results have firmly established the safety and clinical performance of Magmaris, the magnesium-based scaffold could emerge as a strong alternative to currently available polymer-based scaffolds,” commented BIOSOLVE-II principal investigator Dr. Michael Haude of the Lukaskrankenhaus, Neuss, Germany. “Because it is made of magnesium, the scaffold has some unique advantages over polymer-based options in terms of deliverability and radial resistance following the implantation procedure.”
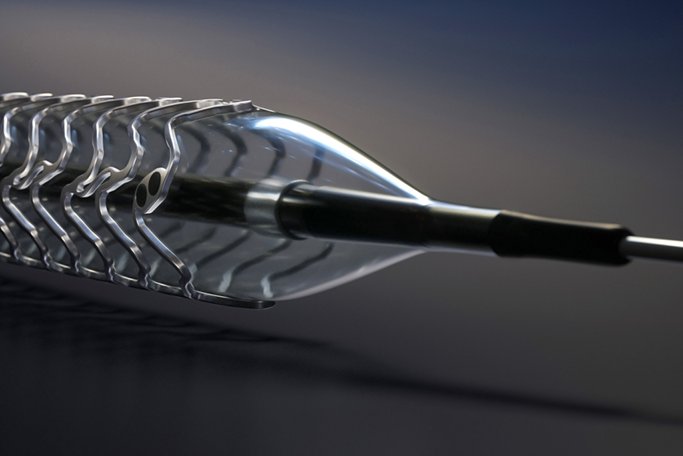
Bench tests show that Magmaris is superior to a leading polymer-based scaffold in terms of deliverability, as it requires 40 percent less force to enter and cross a lesion.1 Physicians will find it easier to steer through vascular anatomy, as 34 percent more force is transmitted to the delivery system end.1 Additionally, Magmaris’s magnesium backbone minimizes recoil following the procedure, meaning that the scaffold is able to withstand external force within the vessel. This ensures the vessel remains open following implantation to prevent potential complications.
In addition to these properties, Magmaris offers a faster resorption compared to polymer-based scaffolds. “The body’s ability to quickly resorb magnesium leads to a faster and therefore more desirable resorption time,” stated Dr. Stephan Kische, Vivantes Cardiology Clinic, Berlin, Germany. “As the results of BIOSOLVE-II demonstrate, vessels can restore vasomotion as soon as six months after the procedure.”2
“CE mark approval for Magmaris opens a new horizon in the vascular therapeutic field,” said Dr. Daniel Buehler, President, Vascular Intervention at BIOTRONIK. “We are eager to bring our magnesium scaffold to market, as we strongly believe that only a resorbable metal alloy can provide patients the distinctive advantages capable of addressing their future needs.”
About Magmaris
Magmaris is a limus-eluting bioresorbable magnesium scaffold exclusively available from BIOTRONIK. Due to the scaffold’s magnesium backbone, it offers novel benefits that only a metallic scaffold can offer such as desired deliverability, strong radial support and a fast resorption time of approximately 12 months. In addition, the proven BIOlute coating, consisting of a limus drug and an excipient, ensures controlled drug release to inhibit cell growth similarly to Orsiro, BIOTRONIK’s hybrid drug-eluting stent.
References
1 BIOTRONIK internal data on file
2 Haude M, et al. Lancet. 2016, 387 (10013)
For more information, visit: www.magmaris.com
Twitter: @BIOTRONIK_News
LinkedIn: www.linkedin.com/company/biotronik