A recent publication discusses long-term results after ATOMS implantation in men with stress urinary incontinence. This study, conducted on 53 men, was published by Dr. Sandra Muehlstaedt from the University Clinic of Urology of Martin-Luther University Halle, Germany. The paper describes peri- and post-operative experience with ATOMS. The demanding criteria for success are as follows: Dryness after ATOMS implantation was defined as less than 10 ml in the 24 h pad test with 0 pads/day or a safety pad; Improvement after ATOMS implantation was defined as less than 25 ml in the 24 h pad test, no more than 2 pads per day, whereby additionally the number of pads has to be reduced by at least 50%. The study included a substantial number of complex cases: 37% of patients underwent radiation prior to ATOMS therapy and 21% of patients had failed therapies prior to ATOMS implantation (e.g. patients after failed AMS 800 artificial urinary sphincter (AUS), or after failed sling implantations of AdVance or Argus).
It is remarkable that the average daily urine loss after ATOMS implantation for this demanding cohort was reduced from 660 ml to less than 25 ml in the 24 h pad test at maximum follow-up for the 77% of patients that were classified as successful. 49% of patients treated with ATOMS were considered dry.
The conclusion of the paper is: "The most influential element of the patient is the patient himself, the degree of incontinence, formerly performed therapies, comorbidities as well as his capabilities and personal preferences. Finally, it is the aim to offer the optimum treatment for the individual patient. In this context, ATOMS offers an effective therapy for treatment of male stress urinary incontinence."
ATOMS - a persuading concept
An interesting study was presented in a lecture at the congress of the German Continence Society in Frankfurt / Main, Germany: Intermediate results of the so-called DOMINO-study (Debates on Male Incontinence).
It is remarkable that this study aims to collect data that is particularly close to reality to assess the medical treatment situation in German speaking countries. The researchers work independently external, visiting and evaluating outcomes in different hospitals. This means that independent analysis of the medical records of the patients are conducted as well as independent patient interviews.
The data presented showed that ATOMS is among the most frequently used implants in this study, which reflects the wide acceptance of ATOMS in Germany and Austria.
ATOMS has shown notably good results in comparison to the AUS, two non-adjustable slings and an adjustable implant. Among all five presented implants, ATOMS has had the best results in the evaluation of "social continence", i.e. 0 or 1 pad/day, i.e. the highest success rate at maximum follow-up.
Additionally, the study assessed pain scores. It is a beneficial for the patients that all implants have shown no pain at maximum follow-up: On a VAS scale from 0 to 10, pain recorded for all implants was below 1, for perineal as well as for inguinal assessment.
In the view of A.M.I., the Domino study provides a very positive description of therapeutic success with ATOMS. The data presented confirms that treatment of male stress urinary incontinence with ATOMS has comparatively very good results. The concept of this implant: it is firmly anchored with mesh arms surrounding the pubic ramus, it incorporates a central silicone cushion of sufficient size for equal pressure distribution, and it offers the ability for a non-surgical post-operative adjustment at any time. The results seem to be a proven concept to treat male SUI.
It didn't come as a surprise that ATOMS was presented to be superior to non-adjustable slings. However, the fact that ATOMS (68% social continence) was even better than the artificial sphincter (52% social continence), was a very positive message for this treatment concept: Especially in view of the fact the artificial urinary sphincter was repeatedly called the gold-standard till recently [1,3]. It is probably the right time to reconsider the term gold-standard in the treatment of stress urinary incontinence in men after radical prostatectomy. The same is requested by Dr. van der Aa (University of Leuven, Belgium) in his recent review of the artificial urinary sphincter [3]: "After 25 years of widespread use, the modern version of the AMS800 AUS has de facto proven to be reliable ... however, the evolution of the therapeutic armamentarium and the current concepts in the field of incontinence management may lead to reconsideration of its gold standard status."
As described by Dr. Muehlstaedt, the individual success of the patient has to govern treatment. ATOMS, with the positive published data in mind, may have its place in such a treatment concept and will hopefully be named in the same breath as a method of choice - in an individualized treatment approach.
ATOMS
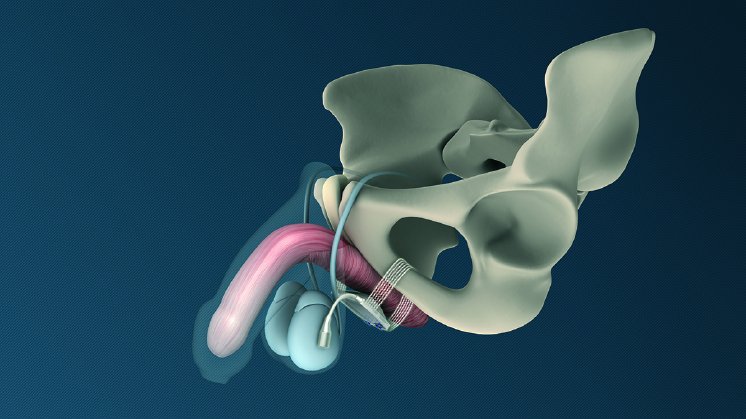
ATOMS (Adjustable Transobturator Male System) is a long-term adjustable implant to treat male stress urinary incontinence after radical prostatectomy.
Designed to allow post-operative adjustment without surgical reintervention, ATOMS comprises a silicone cushion placed under the bulbar urethra and a small-sized access port for adjusting the volume of liquid in the cushion. The concept of ATOMS was developed to allow long-term non-surgical adjustment of the fill volume of the suburethral cushion. Patients have an additional therapeutic option that gives the chance to reduce the loss of urine substantially, as published in various series [e.g. 1,3]. In surgical procedure that is typically performed within 45-60 minutes, the implantation of ATOMS can be conducted in a single-incision approach. Patients and urologists can profit from this innovative approach to treat male stress urinary incontinence. Since its introduction to the market some six years ago, the A.M.I. ATOMS System has been implanted by urologists around the globe multiple thousand times and has achieved high rates of both continence and patient satisfaction worldwide [1,3].
[1] Seweryn J. et al.:
Initial Experience and Results with a New Adjustable Transobturator Male System for the Treatment of Stress Urinary Incontinence., J Urol 2012; 187: 956
[2] Van der Aa F. et al.:
The Artificial Urinary Sphincter After a Quarter of a Century: A Critical Systematic Review of Its Use in Male Non-neurogenic Incontinence, European Urology, Volume 63, Issue 4, Pages 681-689
[3] Primus G., Hoda M.R. et al.:
Early results of a European multicentre experience with a new self-anchoring adjustable transobturator system for treatment of stress urinary incontinence in men., BJU Int. 2013 Feb;111(2):296-303