A critical hurdle has in the past been making enough of the vaccine vector material. This is especially difficult if not impossible when antigens are toxic because they are of viral or bacterial origin as is the case for HIV envelope proteins and bacterial virulence factors. This occurs in the majority of cases.
In a 2-years development project SIRION Biotech applied genetic modification to widespread and inexpensive HEK293 cells and suppressed specific antigen production during the process. This novel cell line overcomes production issues related to all DNA-based viral vectors that are expressing toxic proteins during the process. The technology is generally applicable to the production of DNA-vector based vaccines and can be applied to common vaccine production cell lines.
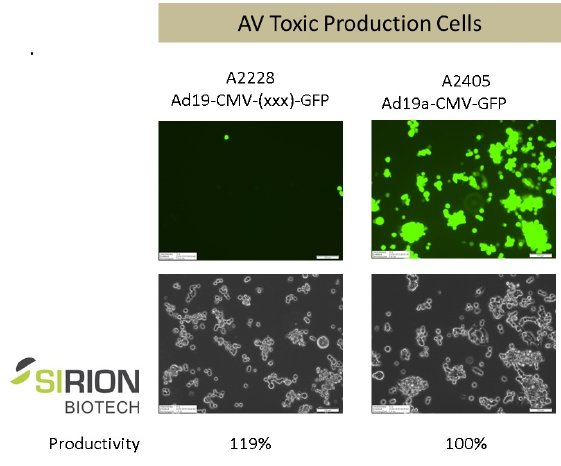
The chart below illustrates the production of Ad19aCMV-GFP in SIRION’s AV-tox cell line with and without regulatory sequences suppressing gene expression of – in this case – GFP, at similar yields.
SIRION Biotech collaborates with Prof. Peter Holst from University of Copenhagen and combines new antigens with his innovative invariant chain adjuvant technology for the benefit of novel cancer immune therapies. Basis of these co-development is SIRION’s lead candidate Ad19a as novel adenovirus serotype with promising immune response-stimulating activity. A treatment vaccine based on this technology against established HPV infections will be evaluated next in a preclinical safety and efficacy study .
Experts gathering for Bio Europe in Munich next week will discuss applications of this novelty.