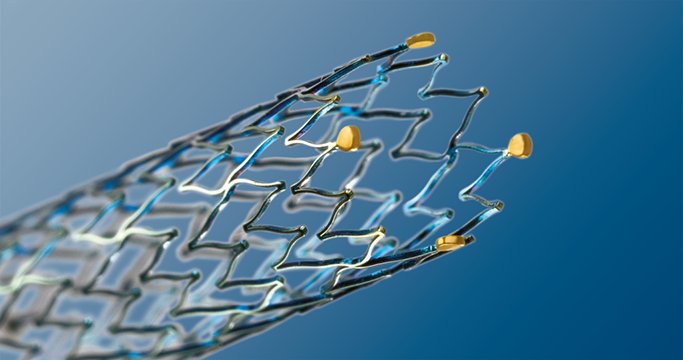
BIOTRONIK and Maquet Medical Systems will deliver a range of BIOTRONIK products used to treat peripheral artery disease (PAD). These include the Passeo percutaneous transluminal angioplasty (PTA) balloon family; the Fortress reinforced sheath; the Cruiser-18 guide wire; and the Astron stent, which was recently granted Food and Drug Administration (FDA) approval for the treatment of iliac artery disease. This is BIOTRONIK’s first FDA approval for a peripheral stent product.
“The Astron stent has been methodically designed for optimal clinical performance in the iliac arteries and is improving the lives of patients worldwide,” said Marlou Janssen, President of BIOTRONIK, Inc. “We are excited to work with Maquet to deliver this and other proven vascular intervention products to patients and physicians in the United States.”
Atherosclerotic disease of the iliac arteries, which supply blood to the pelvic organs and much of the lower limbs, can greatly impact a patient’s overall quality of life, limiting walking ability and leading to chronic pain and even morbidity. Endovascular interventions, including angioplasty and stenting, have become the first-line approach for the treatment of patients with simple and complex peripheral atherosclerotic lesions, including challenging-to-access iliac lesions. In 2014, an estimated 176,800 patients in the United States received an iliac stent, and that number is expected to grow annually.[1]
“The Astron stent is a proven option for treating atherosclerotic disease of the common or external iliac arteries. Results of a 12-month study of Astron demonstrated a Major Adverse Event (MAE) rate of just 2.1 percent, which is remarkably lower than the pre-specified performance goal of 15 percent,” said Dr. Mark W. Burket of the University of Toledo Medical Center in Ohio, and national principal investigator of the BIOFLEX-I IDE study, which evaluated the performance of the Astron stent. “Astron’s 12-month target lesion revascularization rate was also low, at 1.4 percent. Based on these excellent results, I believe that US physicians and patients will welcome this new technology.”
“Peripheral artery disease is a serious condition that can lead to amputations. Because the global prevalence of this disease is increasing, the need for innovative endovascular devices with which to treat patients is growing,” said Raoul Quintero, President of the Americas at Getinge Group, the parent company of Maquet. “Partnering with BIOTRONIK allows us to enhance our current product offerings and expand our existing portfolio of vascular grafts and patches. With the addition of BIOTRONIK’s innovative product line, we can now provide PAD patients in the United States, and the vascular surgeons who treat them, with a complete range of technically-advanced, high-quality endovascular devices.”
More information on the BIOFLEX-I clinical study is available at www.clinicaltrials.gov (identifier NCT01319812).
About Astron
The Astron self-expanding nitinol stent system is designed to provide the specific performance characteristics required for iliac arteries. It features a peak-to-valley stent design with S-shaped articulating connecting bars to provide multi-directional flexibility while avoiding fish-scaling in tortuous arteries. The segmented architecture and strut thickness are engineered to provide optimal chronic outward force. Astron stents are manufactured with BIOTRONIK’s unique proBIO coating, a silicon carbide layer that reduces metal ion release from the stent surface into the surrounding tissue.
About Maquet
Maquet, a trusted partner for hospitals and physicians for more than 175 years, is a global leader in medical systems. The company offers innovative therapy solutions and infrastructure capabilities for high-acuity areas within the hospital - including the operating room (OR), hybrid OR/cath lab, and intensive care unit (ICU) - as well as intra- and inter-hospital patient transport. Headquartered in Rastatt, Germany, Maquet is the largest subsidiary of the publicly listed Getinge Group AB of Sweden.
For more information, visit: www.maquet.com.
References:
[1] Millennium Research Group, 2013. US Markets for Peripheral Vascular Devices 2014, Toronto: Millennium Research Group.