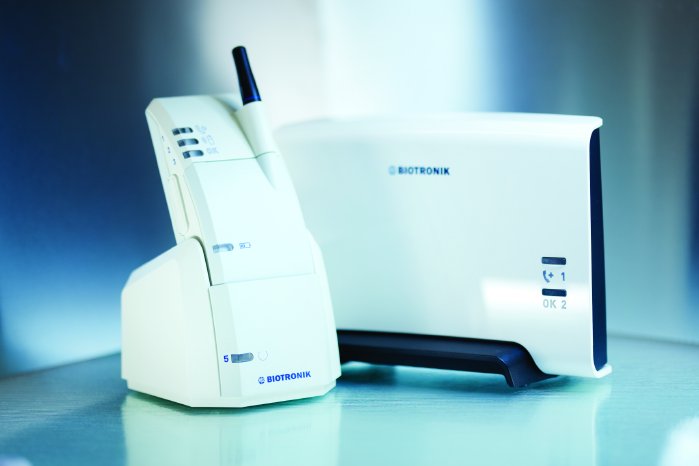
Care of both patient and device after implantation is an essential part of therapy success. Appropriate monitoring of device therapy prolongs a patient's life and enhances its quality. Regular, periodic evaluations are therefore recommended by professional organizations such as the Heart Rhythm Society and European Heart Rhythm Association.[2] Up until now, these evaluations have traditionally been conducted according to fixed, calendar-based appointments every three or six months. Implants with integrated remote monitoring technology transmit medical data as well as technical data about the implant to the physician daily and with full automaticity. This technology helps the physician keep continuous track of a patient's health status and adapt therapy when necessary.
"The TRUST trial demonstrated that automatic remote patient management is more efficient for the clinic. Now, this sub-analysis also shows that Home Monitoring is more effective and reliable in reaching follow-up goals," explained primary investigator Niraj Varma, MD, Cleveland Clinic, Ohio, US. "More frequent conventional, routine in-person evaluations were shown to lead to patient attrition, as patients were perhaps more likely to drop out of the follow-up process when they viewed multiple follow-ups as unnecessary and intrusive."
The TRUST study was a prospective, randomized, multi-center clinical trial that enrolled 1,450 patients at 102 North American sites. It demonstrated the safety and efficacy of BIOTRONIK Home Monitoring® in reducing in-office follow-ups by 45 percent. Now, this sub-analysis shows that remote follow-ups with Home Monitoring also improve patient retention. Within the Home Monitoring group, there was an overall 25 percent greater adherence to all recommended follow-up evaluations. There was also higher adherence to the yearly in-person evaluation when compared with conventional patient management, which was twice as likely to lead to a missed follow-up.
"The TRUST study leads us to believe that we should re-evaluate the traditional standard of care based on in-office evaluation," added Varma. "Perhaps, instead of in-person follow-ups for post-implant patients, the new standard should be automatic, daily remote monitoring, which may lead to more effective patient treatment"
The current guidelines of the European Society of Cardiology (ESC) indicate that a completely automatic and proven remote monitoring system will play an even more important role in the future of device therapy. In fact, ESC has recently recommended implant-based remote monitoring with a grade of IIa and the highest evidence level.
References:
[1] Varma et al., European Heart Journal (3 March 2014). DOI: 10.1093/eurheartj/ehu066.
[2] Wilkoff et al., Europace (2008), 10: 707-725.