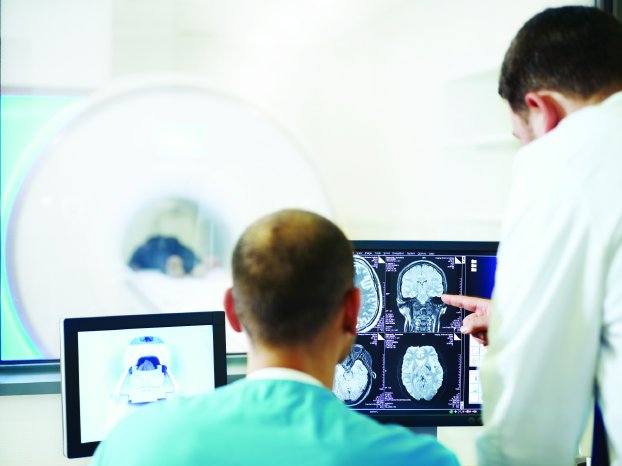
BIOTRONIK is the first company worldwide to gain CE approval for patients with cardiac devices to undergo 3.0 T MRI scans, enabling physicians to receive higher image quality scans more quickly, thereby lessening examination time for patients and making the results all the more useful. Single-chamber and DX implantable defibrillators (ICDs) with atrial diagnostics are now approved with backwards compatibility for ultra high field 3.0 T scans with an exclusion zone.1 Having undergone extensive research and development, BIOTRONIK ICDs that have already been implanted are now approved for use with MRIs.
ICD Patients May Now Undergo Full-Body Scans
BIOTRONIK was the first company worldwide to enable ICD patients to access MRIs in 2011. The company now has CE approval for full-body MRI scans with its single-chamber, dual-chamber and DX ICDs with atrial diagnostics.2 These devices are approved with backwards compatibility for 1.5 T scans. ICD patients are freed from exclusion zones during scans and can benefit from expanded imaging options. In addition, BIOTRONIK remains the only company worldwide to offer heart failure patients with pacemakers and defibrillators (CRT-Ps and CRT-Ds) access to MRI scans.
"Approval for full-body and higher resolution scans offers my ICD patients unparalleled access to potentially life-saving imaging. I often treat cardiac patients with comorbidities who would benefit from MRI diagnostic capabilities," commented Prof. Antonio Curnis, Spedali Civili Hospital, Brescia, Italy. "Now I can be confident in the safety of MRI scans for these patients."
MRI scans have long been the first choice in diagnostics, particularly in soft-tissue imaging. Many cardiac device patients will need an MRI scan within their lifetime,3 as they may suffer from a stroke or comorbidities such as dementia, cancer or arthritis, which are often diagnosed by MRI.
MRI Compatibility Matters
Until recently, patients with a pacemaker or ICD were denied MRI scans due to the strong magnetic fields and radio waves that could negatively influence the devices. While 1.5 T machines remain the clinical standard, 3.0 T MRI scanners improve image quality and reduce scan time. Higher definition MRI scans help physicians see intricate details in soft tissue, for example in the brain. Shorter scan times are helpful for patients who experience claustrophobia or have difficulty holding still, such as young children or adults with dementia. The clinical use of 3.0 T is increasing worldwide,4 further underscoring the importance of MRI scans.
"As a radiologist, it is my job to make sure patients are given access to the best diagnostics to help treat their comorbidities," said Dr. Philippe Cart, Manchester Hospital, Charleville-Mézières, France. "Increased options for BIOTRONIK ICD patients mean that, depending on the clinical indication, patients may safely undergo the scan they need."
About ProMRI®
BIOTRONIK ProMRI® technology enables patients with a pacemaker, implantable defibrillator, or cardiac resynchronization therapy defibrillator (CRT-D) or pacemaker (CRT-P) to undergo an MRI scan. BIOTRONIK has the broadest portfolio of cardiac devices approved for use with MRIs on the market and is the only company to allow heart failure patients with CRT devices to take advantage of MRI scans. ProMRI® technology enables pacemaker patients, and now ICD patients, to undergo full-body scans. ICD patients may now also undergo ultra high field 3.0 T scans.
References:
1 3.0 T scans are approved for Ilesto, Iforia, and Idova single-chamber and DX ICDs with LinoxSmart and Protego ProMRI® leads with an exclusion zone.
2 Full-body 1.5 T scans are available with Ilesto and Iforia single-chamber, dual-chamber and DX ICDs with Safio S and LinoxSmart ProMRI® leads.
3 Kalin and Stanton, Pacing and Clinical Electrophysiology (2005), 28: 326-328.
4 Data on file.