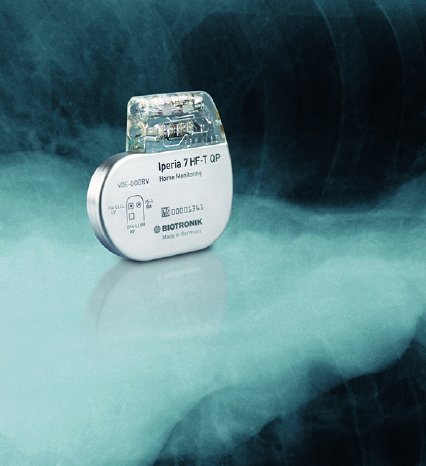
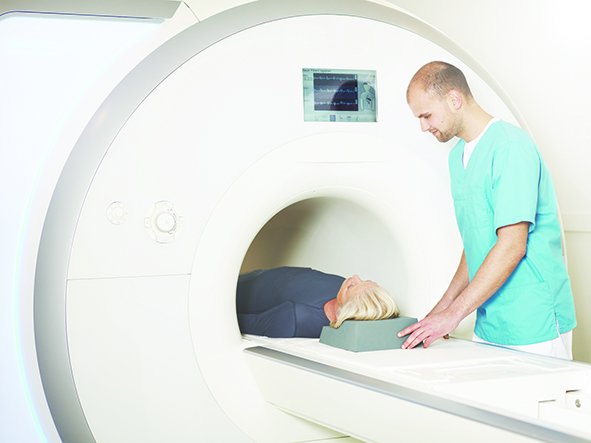
With its ProMRI® technology, BIOTRONIK is the first and only company to offer heart failure patients CRT-Ds and leads that can undergo MRI scans. Patients with single-chamber ICDs can take advantage of 3 tesla MRI scans with an exclusion zone. Both single- and dual-chamber ICD patients are eligible for 1.5 T full-body scans. To easily track the MR conditions of implantable devices, BIOTRONIK has recently launched ProMRI® SystemCheck.
Dr. Klaus-Jürgen Gutleben, physician of internal medicine and cardiology at the Heart and Diabetes Center North Rhine-Westphalia, Germany, is looking forward to offering his patients CRT solutions approved for MRIs. His clinic implants 200-300 patients with CRT devices every year. "MRI diagnostics are very important for my younger patients, who are better off avoiding radiation from X-rays or CT scans and patients with comorbidities like brain tumors, which are best diagnosed with high-resolution images," said Dr. Gutleben. "For such patients, I would recommend an implant that can undergo MRI scans, such as the Iperia CRT-D and the Sentus QP lead."
The Sentus QP lead eases the implantation process by giving physicians better access to challenging vessels. Sentus QP represents the industry's first quadripolar left-ventricular lead to be approved for MRI use. It offers stable lead positioning in the coronary sinus and various electronic repositioning options to select the optimal stimulation site.
"When deciding on the right device for my heart failure patients, I want to make sure they will benefit as much as possible from cardiac resynchronization therapy," explained Dr. Gutleben. "Clinical evidence demonstrates that patients have reduced mortality when they receive fewer inappropriate shocks. We also know it can greatly improve their mental well-being and satisfaction with the therapy. Detection criteria and morphology analysis help ensure my patients only receive the therapy they need."
The new ICDs reduce inappropriate shocks with MorphMatch morphology detection criteria and optimized anti-tachycardia pacing (ATP), making it easier to give a patient exactly the level of pacing therapy he or she might need. While delivering shocks at the right time can save patients' lives, minimizing shocks improves patients' quality of life.
"Our engineers working in R&D are dedicated to constantly improving and innovating state-of-the art technology. They take into account physicians' and patients' needs, creating solutions that set new standards that are clinically proven. IN-TIME is one such study, demonstrating that BIOTRONIK Home Monitoring® reduced mortality of heart failure patients by more than 50 percent,"[1] said Wolf Ruhnke, Vice President at BIOTRONIK. "The new ICD and CRT-D series is the result of 50 years of innovation and an unwavering sense of responsibility when it comes to helping patients live normal lives."
References:
[1] Hindricks G et al. The Lancet. 2014, 384(9943).