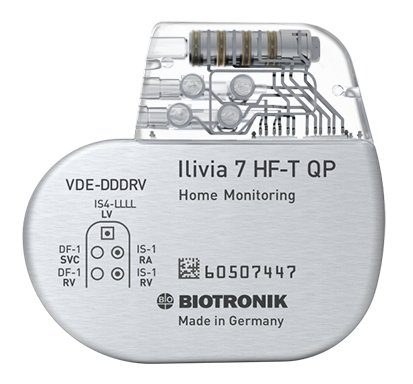
At present, when a cardiac device patient undergoes an MRI scan, a cardiologist must program his or her device into a special mode that temporarily reduces the device’s functionality until the MRI scan is completed. Following the scan, the cardiologist must switch off the device’s MRI mode to allow the patient to once again have the full therapeutic benefits of their implanted device. MRI AutoDetect allows the cardiologist to activate a special window, lasting up to 14 days, in which all device functionality is maintained until the patient is undergoing the scan itself. At that point, the device switches into MRI mode automatically. Once the scan is completed, all device functionality is restored, again automatically and without the need for the cardiologist’s intervention. Soon after the scan, the cardiologist receives a full report via transmission through BIOTRONIK Home Monitoring®.
“It is only in the last few years that cardiac device patients could safely undergo MRI scans at all. However, even now, patients aren’t fully protected if they have an event while their devices are programmed in MRI mode—a period that currently can last a day or more depending on hospital workflows,” commented Dr. Richard Kobza, Head of Cardiology, Luzerner Kantonsspital, Lucerne, Switzerland. “Reducing the amount of time a device is in MRI mode is particularly crucial for ICD and CRT-D patients, and with MRI AutoDetect the only time these patients won’t be able to benefit from full device therapy is the short 30-minute window they spend in the MRI machine itself.”
“MRI AutoDetect is the next step in ensuring that patients get the full benefit of both their implanted devices and diagnostic imaging,” commented Manuel Ortega, Senior Vice President at BIOTRONIK. “MRI AutoDetect will not only reduce the amount of time the patient’s device stays in MRI mode, but I expect it will also greatly improve the workflow between the cardiologist and radiologist, which will ultimately benefit our patients.”
Ilivia devices also feature MultiPole Pacing (MPP) to further improve therapy for CRT patients, the company’s unique Closed Loop Stimulation (CLS) algorithm for more adaptive pacing and DX technology for ICDs and CRT-Ds.
About ProMRI
BIOTRONIK ProMRI technology enables patients with a pacemaker, implantable defibrillator, cardiac monitor, or cardiac resynchronization therapy defibrillator (CRT-D) or pacemaker (CRT-P) to undergo an MRI scan. Devices with MRI AutoDetect have the additional capability of automatically recognizing an MRI environment within a programmable time window, switching on the device’s MRI mode for only as long as is required to complete the scan. This typically lasts around 30 minutes. This ensures the patient receives the full benefit of their device for the maximum amount of time possible. BIOTRONIK has the broadest portfolio of cardiac devices approved for use in the MR environment on the market. For more details, please go to www.biotronik.com/promri.